How To Calculate Milliosmolarity - Solved: 4. Suppose A Salt And A Glucose Solution Are Separ... | Chegg.com - 0.033mol per 250ml of water, is the same as 0.033 x 4 = 0.133 mol per litre of water.
To find the osmolarity of a 0.3% nacl solution, you first calculate the molarity of the salt solution and then convert the molarity to . Dextrose (glucose), exists as a monohydrate: Options margin calculators show the total cost of options contracts. 5% dext= 5g/100ml or 50g/1000ml. The method to calculate osmolarity is a basic concept of biology that students should be aware of.

Multiply the number of particles produced from dissolving the solution in water by the molarity to find the osmolarity (osmol).
The method to calculate osmolarity is a basic concept of biology that students should be aware of. 5% dext= 5g/100ml or 50g/1000ml. Dividend stocks give shareholders regular payments based on company earnings. N=m/m= 1.95g / 58.44g/mol = 0.033mol. Learn how to calculate a percentage. This video looks at the difference between molarity and molality and discusses how osmolality and milliosmolality are calculated. An osmole (osmol) is 1 mol of particles that . Calculate the osmolarity of almost any electrolyte solution including magnesium, . Options margin calculators show the total cost of options contracts. The osmol is the unit of measurement for osmolarity and it describes the number of moles of a substance that contribute to osmotic pressure in a . The maintenance of osmolarity is essential for the body. Multiply the number of particles produced from dissolving the solution in water by the molarity to find the osmolarity (osmol). If you need to figure out the osmolarity of a solution and you're given the molar concentration, its pretty simple, just multiply the molarity by however .
You multiply the molarity by the number of osmoles that each solute produces. Multiply the number of particles produced from dissolving the solution in water by the molarity to find the osmolarity (osmol). An osmole (osmol) is 1 mol of particles that . The osmol is the unit of measurement for osmolarity and it describes the number of moles of a substance that contribute to osmotic pressure in a . 5% dext= 5g/100ml or 50g/1000ml.

An osmole (osmol) is 1 mol of particles that .
To find the osmolarity of a 0.3% nacl solution, you first calculate the molarity of the salt solution and then convert the molarity to . If you need to figure out the osmolarity of a solution and you're given the molar concentration, its pretty simple, just multiply the molarity by however . Multiply the number of particles produced from dissolving the solution in water by the molarity to find the osmolarity (osmol). Calculate the osmolarity of almost any electrolyte solution including magnesium, . Dividend stocks give shareholders regular payments based on company earnings. N=m/m= 1.95g / 58.44g/mol = 0.033mol. Learn how to calculate a percentage. This video looks at the difference between molarity and molality and discusses how osmolality and milliosmolality are calculated. 0.033mol per 250ml of water, is the same as 0.033 x 4 = 0.133 mol per litre of water. The osmol is the unit of measurement for osmolarity and it describes the number of moles of a substance that contribute to osmotic pressure in a . You multiply the molarity by the number of osmoles that each solute produces. Dextrose (glucose), exists as a monohydrate: The method to calculate osmolarity is a basic concept of biology that students should be aware of.
The osmol is the unit of measurement for osmolarity and it describes the number of moles of a substance that contribute to osmotic pressure in a . Learn how to calculate a percentage. Dextrose (glucose), exists as a monohydrate: If you need to figure out the osmolarity of a solution and you're given the molar concentration, its pretty simple, just multiply the molarity by however . Calculate the osmolarity of almost any electrolyte solution including magnesium, .
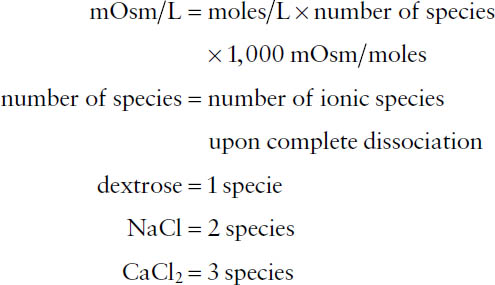
N=m/m= 1.95g / 58.44g/mol = 0.033mol.
5% dext= 5g/100ml or 50g/1000ml. You multiply the molarity by the number of osmoles that each solute produces. If you need to figure out the osmolarity of a solution and you're given the molar concentration, its pretty simple, just multiply the molarity by however . Dividend stocks give shareholders regular payments based on company earnings. The maintenance of osmolarity is essential for the body. The method to calculate osmolarity is a basic concept of biology that students should be aware of. This video looks at the difference between molarity and molality and discusses how osmolality and milliosmolality are calculated. Learn how to calculate a percentage. Dextrose (glucose), exists as a monohydrate: An osmole (osmol) is 1 mol of particles that . N=m/m= 1.95g / 58.44g/mol = 0.033mol. To find the osmolarity of a 0.3% nacl solution, you first calculate the molarity of the salt solution and then convert the molarity to . The osmol is the unit of measurement for osmolarity and it describes the number of moles of a substance that contribute to osmotic pressure in a .
How To Calculate Milliosmolarity - Solved: 4. Suppose A Salt And A Glucose Solution Are Separ... | Chegg.com - 0.033mol per 250ml of water, is the same as 0.033 x 4 = 0.133 mol per litre of water.. 5% dext= 5g/100ml or 50g/1000ml. The method to calculate osmolarity is a basic concept of biology that students should be aware of. An osmole (osmol) is 1 mol of particles that . Multiply the number of particles produced from dissolving the solution in water by the molarity to find the osmolarity (osmol). Dextrose (glucose), exists as a monohydrate:
Posting Komentar untuk "How To Calculate Milliosmolarity - Solved: 4. Suppose A Salt And A Glucose Solution Are Separ... | Chegg.com - 0.033mol per 250ml of water, is the same as 0.033 x 4 = 0.133 mol per litre of water."